Keith Krehbiel was just 42 when he was diagnosed in 1997 with early-onset Parkinson’s disease. At first it had been a sore hand that concerned him. In time he realized his pinkie finger often trembled. On the drive home from the doctor’s office, he stopped at the Stanford Shopping Center to pick up a book on the disease that his physician recommended, even before he told his wife. He wanted to know more to help calm his fears.
“I wasn’t scared I was going to die soon, but I did want to know if I was going to die sooner,” he says. “My goal was to live long enough for my daughters to graduate from college and high school.”
Parkinson’s—a progressive neurological disorder without known cause or cure—may shorten lifespan in severe or untreated cases, but there’s no medical consensus about its effect on lifespan. While the disease’s progression varies from person to person, it tends to advance more slowly in early-onset patients. For the first two decades after he was diagnosed, Krehbiel, now a professor emeritus at the Graduate School of Business, took medications to treat his growing symptoms—the tremors, the rigidity, and the imbalance that resulted in three emergency room visits, two of them after he fell on campus and one after a fall at home. He stopped driving a car, sensing his wife’s concern for him, and began bicycling to the GSB. When two-wheeled transport grew precarious, he bought a more stable recumbent tricycle.
 Photo: Kim Ratcliff
Photo: Kim Ratcliff
He stopped driving a car, sensing his wife’s concern for him, and began bicycling to the GSB. When two-wheeled transport grew precarious, he bought a more stable recumbent tricycle.
“I did get to see my daughters graduate, and now I’ve got three grandchildren,” he says.
But, as often happens in Parkinson’s patients, Krehbiel’s medications became less and less effective over time at controlling his motor symptoms. As the medications wore off, his head would bob and weave—one of a category of involuntary movements known as dyskinesia. And when he increased his doses, he experienced nausea and brain fog. For a professor, that changed the calculation. He decided it was time to pursue a surgical approach to managing his symptoms—namely, deep brain stimulation (DBS).
DBS is a technique developed in the 1980s and 1990s in which a surgeon inserts electrodes deep into areas of the brain that are affected by Parkinson’s, then connects them to a small, battery-powered device implanted in the chest that sends electrical pulses back to the brain. The stimulation helps modulate the electrical activity in the targeted brain area, reducing abnormal brain signals and, thus, symptoms. Krehbiel reached out to a neurologist who was conducting research on DBS: Helen Brontë-Stewart, a professor of neurology and neurological sciences who has spent much of her career seeking to understand, measure, and improve the brain’s control of movement in the body.
At the time, Brontë-Stewart was leading a multisite global clinical trial for a new and improved version of DBS called adaptive DBS (aDBS). In the original DBS system, the neurostimulator sends a steady stream of electrical pulses to either the subthalamic nucleus or the globus pallidus internus, two brain structures in the basal ganglia key to motor control. But with Parkinson’s, patients’ motor symptoms change as they go through their daily activities, from waking to walking to driving a car, and as the effects of medication wax and wane. So, the aDBS neurostimulator varies the amplitude of the electrical charges it sends to the basal ganglia in keeping with the patient’s need for them. “Like a cardiac pacemaker that responds to the rhythms of the heart, adaptive deep brain stimulation uses a person’s individual brain signals to control the electric pulses,” Brontë-Stewart says. “This makes it more precise and more efficient than older DBS methods.” The treatment didn’t promise to cure the disease; instead, the goal was to improve quality of life, which, for Krehbiel, included his ability to play with his grandchildren and to continue his research on legislative politics.
Krehbiel signed up for the trial and, in 2020, after two months of DBS treatment, he became the first person to receive experimental treatment with aDBS. “He’s a special case,” Brontë-Stewart says. “He’s been on adaptive DBS longer than anybody in the world. ” Krehbiel acknowledges the fortune more than the fear in that fact. “I mean, the technology kind of blows me away, but the process doesn’t strike me as being extraordinarily risky,” he says. “I don’t feel like I deserve any heroic status or anything for getting in line first and doing it.”
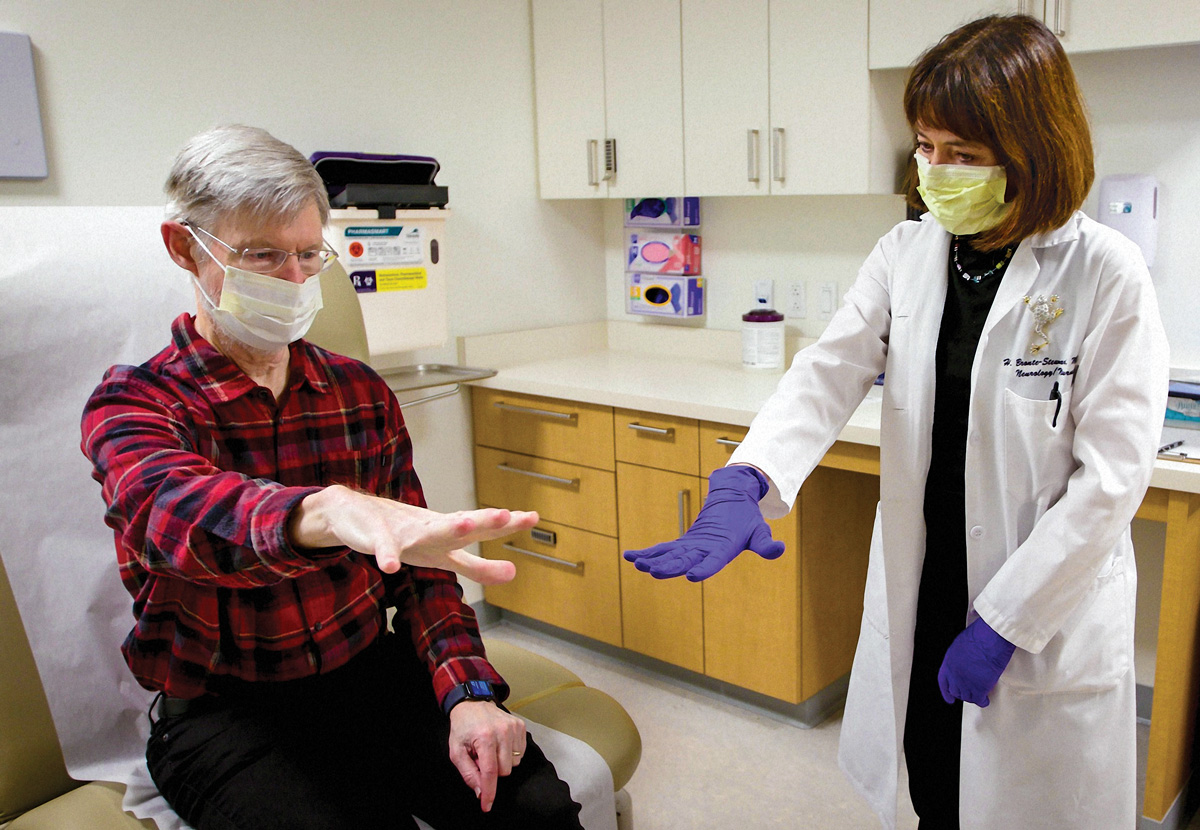 HELPING HANDS: Brontë-Stewart sees Krehbiel regularly in the clinic and monitors his symptoms closely. (Photo: Todd Holland)
HELPING HANDS: Brontë-Stewart sees Krehbiel regularly in the clinic and monitors his symptoms closely. (Photo: Todd Holland)
‘He’s a special case. He’s been on adaptive DBS longer than anybody in the world.’
In the trial, surgeons at 10 sites worldwide connected participants with existing electrode implants to the new and improved neurostimulator. Brontë-Stewart’s lab focused on understanding how the device needed to respond to the brain—and enabling it to do so. “Our contribution was to identify these relevant neural signals, then develop the algorithms used to determine when and at what levels to send out the electrical charges,” she says. In February, the FDA, which had access to data from the primary cohort of 68 patients, approved the use of aDBS, delivered via a state-of-the-art Medtronic neurostimulator, for people with Parkinson’s.
Although the trial results have not yet been made public, participants have told Brontë-Stewart that they experienced fewer side effects with aDBS than they had with the original DBS treatment, also called continuous DBS because it doesn’t modulate its levels of stimulation. They also reported that aDBS provided more effective therapy than continuous DBS did.
For Krehbiel, it’s clear that deep brain stimulation—potentially both approaches—has helped, even as some symptoms persist or worsen. “My tremor is much better—all but gone,” he says. He still falls frequently due to low blood pressure and poor balance. He can’t make the nuanced facial expressions he’d like to, which affects his social interactions. But, he says, “I was able to reduce my medication significantly, and the nausea and brain fog are gone. Consequently, in my day-to-day life, my attitude is just better.”
The innovator
Before Brontë-Stewart became a neuroscientist, she was a ballet student growing up in Glasgow, Scotland. At 14, she took a Royal Academy of Dance exam as she prepared to one day audition for the Scottish Ballet training school. She well remembers that exam. The instructors, their white hair pulled back in tight buns, stood before her moving their hands in complicated motions. The dancers were expected to mimic the motions with their feet, and they could do so immediately and without thought. Brontë-Stewart passed the exam, then headed home, pondering.
“On the train ride home, I began to wonder how it was possible to do this,” she says. “How did my brain know how to move my feet without me thinking about it?” It’s a question that has guided her career.
The brain is a supremely complicated electrical organ with about 86 billion nerve cells. These nerve cells, or neurons, are like current-carrying wires. They interact and link together to create distinct brain regions with specific functions and complex circuits that control movement. In a healthy brain, a movement request begins in the command center in the cortex, gets sent through the basal ganglia to the midbrain along circuits that connect to the brainstem, then to the spinal cord, and, finally, to the nerves that connect to the muscles, resulting in a leg lifting or an arm waving. Movement disorders such as Parkinson’s disease reflect disordered electrical activity in circuits in the basal ganglia. Within that region, dopamine-producing cells die off and the levels of dopamine—a neurotransmitter—become inadequate to send movement signals.
Brontë-Stewart followed her curiosity by earning a bachelor’s degree in physics and mathematics at the University of York, then a master’s in bioengineering, a medical degree, and a neurology residency, all at the University of Pennsylvania. During a fellowship at UCSF, she trained in making microelectrode recordings of the neural circuits in primate brains. Afterward, she went to work as a neurologist at Kaiser Permanente in San Rafael, Calif. Soon, she made a connection that sped her path to DBS.
The modern era of deep brain stimulation was underway, and researchers—led, in the United States, by Emory University—were uncovering precise spots within the brain to target for Parkinson’s treatment. Neurologist Jerrold Vitek, now chair of neurology at the University of Minnesota, was on the Emory team that first used electrical stimulation to target the globus pallidus and subthalamic nucleus in the basal ganglia for DBS.
“I met Jerry Vitek at a medical conference, and we sat down on the floor and started mapping out brain circuits,” Brontë-Stewart says. Invigorated, she asked if she could come to Emory to study their methods. The Emory team agreed, and she began making training trips to Atlanta. “With her background in electrophysiology, she learned the process very quickly and understood the methods and approach we had developed,” Vitek says. “She wanted to learn how we mapped, how we used the physiological recordings to determine where we were in the brain to define where to make the lesion. She was so excited about these surgeries.”
When Brontë-Stewart participates in DBS surgeries, it’s as if the patient’s brain is talking to her. The different structures make varying sounds from which she can build a 3-D image of the patient’s brain that helps her provide input to the surgeon.
Brontë-Stewart used her training at Emory in DBS to help set up the first surgical center for movement disorders at Kaiser. Then, in 1999, she was recruited to Stanford to help establish the movement disorders clinic and the DBS surgical program.
Brontë-Stewart launched her new lab at Stanford with an effort to develop tools to quantitatively measure motor symptoms in patients with Parkinson’s. She believed this was the first step toward understanding which brain signals were relevant to abnormal movement, knowledge that would help them build a better DBS system. One of her early tools, called quantitative digitography, involved patients alternating tapping their index and middle fingers on two custom-engineered piano keys attached to a spring. The device then recorded measurements of amplitude and timing.
Today, the technology has been developed as a remote-control device that patients can use at home to provide real-time metrics about the changes in their motor symptoms, via an iPhone, to health care providers. Brontë-Stewart took a partial sabbatical last summer and fall to help it earn an FDA Breakthrough Device designation, and this summer she did the same to help move the device further along its regulatory path.
“For my patients who have used it, they can come into the clinic, and I already know their motor function,” Brontë-Stewart says. “The health care provider can monitor them and intervene when they see a problem, rather than waiting the typical three to six months between appointments.”
The focus on measurement laid the groundwork for aDBS. In 2013, Medtronic developed a neurostimulator that, in addition to targeting brain signals, could record them—a sort of “brain radio,” Brontë-Stewart calls it. Previously, the only time neural signals deep in the human brain could be recorded was during surgery while the brain was open. Using Medtronic’s new design, Brontë-Stewart’s lab became the first in the United States to collect data from brain recordings made in clinic while patients were asked to do different tasks, such as walk around, stand up, or sit down.
“So, we have a person who can move, but their brain is telling them to move abnormally,” Brontë-Stewart says. “We needed to be able to record the arrhythmias, then write some algorithms to return them to normal.” Being able to control the amplitude of the stimulation that the neurostimulator emitted in real time—that would be key.
“Once we had figured out the relationships between symptoms and brain signals, we were able to create algorithms based on the incoming brain signals that would determine how stimulation should change, increasing or decreasing the stimulation as needed,” says Kevin Wilkins, a research scientist in Brontë-Stewart’s lab. That technology is embedded in the aDBS neurostimulator.
The updated device can respond dynamically and in real time, like a pacemaker. For Brontë-Stewart, the new system is something of a lifetime of work come to fruition. Most importantly, it may mean better treatment for her patients who choose to have brain surgery as a treatment for Parkinson’s.
The procedure
The lights are dimmed in the Stanford operating room when Brontë-Stewart enters, wearing blue scrubs. A neurosurgeon has just finished drilling two holes into either side of his patient’s skull and has threaded two thin wires with electrodes down each hole toward the subthalamic nucleus. It’s essential to get the electrodes positioned on that exact spot, an area roughly the size of a pencil tip. Brontë-Stewart’s role is to help guide the surgeon in his placement of the electrodes. It’s a skill, honed over decades, that she now teaches to her neurology trainees. Not every center uses neurologists to help during DBS or aDBS surgery (the surgeries are the same), but Stanford almost always does—the surgeons insist it improves outcomes due to more exact placement of the electrodes.
(“This is when the magic happens,” Brontë-Stewart says later in an interview.)
Brontë-Stewart moves around the surgical bed, giving instructions for the patient to think about while she moves his fingers, elbows, thighs, feet. “Now move your leg up and down, up and down,” she says, all the while listening intently to the corresponding sounds coming through her headset.
“I’m literally mapping the brain, moving the arms and legs and calling out what I’m hearing to the surgeon,” Brontë-Stewart says. “If you move the limbs, you get a sensory volley. That means the brain is firing away. It makes a noise that’s picked up by amplifiers. If you know what you’re listening to, it can really sound like a melody.
“For me, it’s just beautiful to listen to the brain,” she says. “It’s such a privilege. I mean, how could you ever imagine listening to the brain and helping somebody?”
Professor of neurosurgery Jaimie Henderson has performed hundreds of these surgeries. “After drilling two holes in the skull, I use computer guidance to find the best spot in the target region,” he says. “We also check from the recordings of the neurons, listening to those brain cells. Helen helps us to interpret those signals.” The surgeons link Brontë-Stewart’s—or another neurologist’s—interpretations or directions with the real-time imaging of the brain.
“You have to have someone really good at this to verify the location,” Henderson says. “Helen is one of the world’s best.”
Brontë-Stewart says being part of DBS therapy from the beginning has been one of the most rewarding aspects of her career. “It starts in the operating room when the person’s tremor rigidity and bradykinesia melt away,” she says. “Some patients start to cry; others look at me in amazement that their symptoms could go away in a flash. I get to be the person right up close to experience this with them in real time and to assure them that this is a sign of the improvement to come.”
Krehbiel remembers coming out of the anesthesia and listening to Brontë-Stewart’s commands. “They put me to sleep; they drilled holes in my head, and then before they sewed me up, they woke me up. So I’m lying there waking up, and Helen is there. She was awesome. She just took charge,” he says. Today, he leans forward to show beneath his hair the outline of the two holes, now capped, that his surgeon drilled into his head. Then he traces the electrode wires snaking from his brain along his neck. He can feel them ducking under his collarbone as they extend a few more inches to the neurostimulator’s battery pack in his chest, its perimeter almost that of a playing card’s.
Krehbiel still sees Brontë-Stewart regularly in the clinic. She has monitored his symptoms closely since his aDBS treatment and documented the results. These days, he worries about the specter of cognitive decline, a symptom of Parkinson’s that tends to develop later in the progression of the disease. Available treatments—mainly medications meant for Alzheimer’s patients—aren’t very effective for Parkinson’s, but Brontë-Stewart has launched a pilot study targeting circuits in the brain that her lab has shown are linked with cognitive decline. Perhaps that will benefit the next generation of patients.
Over the five years since his surgery, some of Krehbiel’s symptoms have worsened. He realizes that his illness will continue to progress. But his quality of life is better, he says, because of aDBS. “I think that’s going to be the case over the course of my remaining life. I’ll be better off with it than I would have been without.”
Tracie White is a senior writer at Stanford. Email her at traciew@stanford.edu.



